Cycling and Education (CLEAT)

*UPDATE*
We are pleased to confirm that the CLEAT study has now completed recruitment. Two hundred and twenty-one participants were enrolled on to the study. Owing to a lower drop-out rate than expected we were able to recruit slightly less than our original target of 256.
We are pleased to now share the results of the CLEAT study. You can read the publication here - https://www.thelancet.com/journals/lanrhe/article/PIIS2665-9913(25)00102-X/fulltext, and you can find the press release here - Orthopaedic Research Institute | Cycling and Education Programme (CHAIN) Outperforms Traditional Physiotherapy in Major Study.
What is the CLEAT study

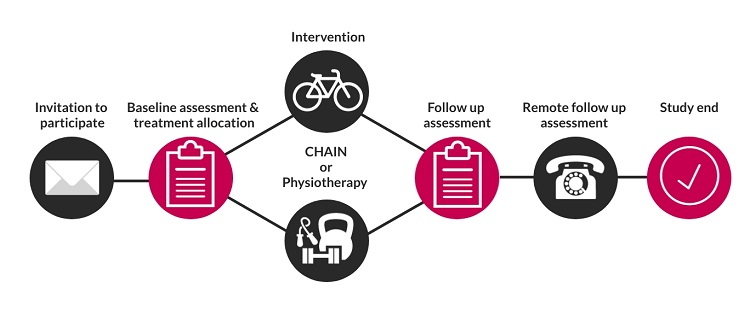
The CHAIN intervention was developed as a group education and static cycling programme, to equip participants with the confidence to self-manage their condition and increase their ability to do daily activities. The aim of this study is to investigate whether CHAIN is more clinically effective and cost-effective than routine physiotherapy care for patients with osteoarthritis of the hip.
What are the benefits of taking part in the study?
This research aims to improve patient care. By taking part in this research, you will help to guide treatment for future patients. In addition to your treatment, you will also benefit from a baseline and follow up assessment with a physiotherapist, where you will be able to ask further questions and measure the outcome of your treatment.
Am I suitable for the study?
You may be considered suitable if you are aged 18 years or older, have hip pain or hip arthiritis, and:
- Haven’t had hip surgery within the last 6 months
- Are not planning on having lower limb or back surgery in the next 9 months
- Haven’t used oral or intra-articular corticosteroids in the last 3 months (except for asthma)
You should not take part if you are:
- Pregnant and not exercising regularly
- Unable to get on or off a bike.
How can I take part in the CLEAT study?
- You can refer yourself - This can be done via the Musculoskeletal (MSK) Dorset website https://www.mskdorset.nhs.uk/patient-self-referral-form/, and stating that you would like to be considered for the CLEAT study
- You can be referred by your GP, Consultant, or physiotherapist - This can be done by asking your GP or Health Professional to refer you to the physiotherapy department at Christchurch Hospital, University Hospitals Dorset, stating that you are interested in taking part in the CLEAT study
How can GPs and other clinicians refer patients to the CLEAT study?
Please refer your patients to physiotherapy at Christchurch Hospital, University Hospitals Dorset, stating that they may be suitable for taking part in the CLEAT study.
What happens once I have been referred?
Once we have received your referral, you will be contacted by telephone to discuss the study and check you are suitable to take part. If you choose to take part, you will be invited to attend a baseline assessment at the Orthopaedic Research Institute, Bournemouth University.
Study research team
Chief Investigator: Professor Tom Wainwright
Trial Managers: Tikki Immins and Lauren Daughtrey (please contact This email address is being protected from spambots. You need JavaScript enabled to view it. or 01202 962727
Clinical Trial Assistants: Chloe Bascombe, Nhi Dao
Clinical Assessment Team: Jo Garofalides, Harriet Legg, and Ellie Whale
Clinical Team: Matt Low, James Creasey, Liam Johnson, Jo Masterman, Emily Dore-Smith
Where is the study delivered?
The study is delivered in Bournemouth, Dorset, in England. All assessments take place at the Orthopaedic Research Institute, at Bournemouth University. The CHAIN programme is delivered at the Littledown Centre, opposite The Royal Bournemouth Hospital. Physiotherapy care is delivered at Christchurch Hospital.

How do I take part in the study?
Participants are considered for the study if they have been referred to the Physiotherapy Department at University Hospitals Dorset for the treatment of hip pain. Potentially eligible participants will then be contacted by Chief Investigator, Professor Tom Wainwright, to discuss participation within the study.
Can I choose which treatment arm I am in?
No, it is not possible to choose which treatment arm you are in. Treatment arms are allocated randomly using online software. To ensure the study is fair, we cannot let you choose which treatment you receive, or change your treatment arm once it has been allocated. Both treatment arms have proven successful in managing the symptoms of patients with hip osteoarthritis.
Will I be paid for taking part?
You will not be paid for your participation in this study. You will not incur any expenses above that of standard care. All ongoing care that might be required will be provided by the NHS.
Can I withdraw from the study?
You can withdraw from participation in the study at any time without giving a reason. If you withdraw, we will use all of the information that we have collected about you up to that point.
What will happen at the end of the study?
The results from this study will be used to inform and influence decisions around care for hip osteoarthritis in the future. The findings will be published in relevant scientific journals and presented at conferences and meetings. No personal identifiable data will be disclosed in any publication. The results from the study will also be made available on the Orthopaedic Research Institute’s website https://microsites.bournemouth.ac.uk/ori/ and the UHD’s Research and Innovation (R&I) website.
Who is organising and funding the research?
The National Institute for Health Research (NIHR) – Research for Patient Benefit, are funding this study It is organised by the Orthopaedic Research Institute, at Bournemouth University, in collaboration with the Research and Development department at University Hospitals Dorset.